-40%
FDA Approved Fully Automatic Upper Arm Blood Pressure Monitor 3 mode 4 Cuffs USA
$ 34.31
- Description
- Size Guide
Description
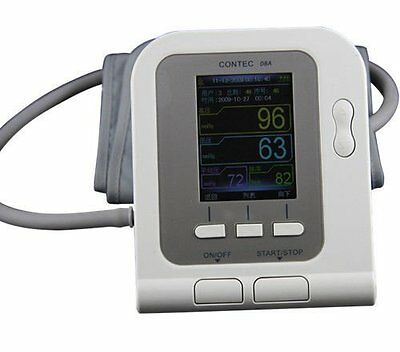
US LCD Digital Blood Pressure Monitor,Software,(Adult,Child,Infant,Neonate) CuffIntroduction
CONTEC08A is a desktop Electronic Sphygmomanometer,High-definition color LCD display,supply English/Chinese interface,Strong visibility.Fully automatic blood pressure measure,it can be a household sphygmomanometer which is used for adult, pediatric and neonatal.The device has the function of parameter measure,display and record output,and adopt data review interface such as "data list", "trend chart", "big font",with SpO
2
measure function(with optional SpO
2
probe).It can be applied to the clinique,physical center and daily health care of family member.
Features
Fully automatic blood pressure measure
The Electronic Sphygmomanometer stores the measure results of three users automatically, and up to 100 items for every user.It can satisfy blood pressure measure requirement of all your family.
Three kinds of measure modes : adult, pediatric and neonatal.
By data review interface such as "data list","trend graph","big font", NIBP data is clear at a glance.
With SpO
2
measure function(with optional SpO
2
probe).
Has the function of physiological alarm.It can be set the alarm limits,when blood pressure is higher than the high limit or lower than the low limit, the physiological alarm will occur.Alarm switch can be set.
Screen displays prompt message when the power is low,and the device gives low power prompt sound.The prompt sound switch can be set.
When there are factors which affect the measure in the process of measure and the device can't get the measure result,the device can display the corresponding error message.
With short-time power storage function, when replacing the battery, the clock time function can't be affected.
Supply two kinds of NIBP measure unit: mmHg / kPa
Store measure results with date and time.
High-definition color LCD display,supply English/Chinese interface, strong visibility.
Communicate with PC,PC software can achieve data review, analysis measure results, seeing trend, printing reports and other functions.
Function of automatic power-off.
Performance
NIBP
Measure Method: Oscillometry
Measure Mode: The upper arm measure
Measure range: 0kPa(0mmHg)~ 38.67kPa(290mmHg)
Resolution: 0.133kPa(1mmHg)
Accuracy: ±0.4kPa(±3mmHg)
NIBP pulse rate measure range: 40~240bpm
Increasing pressure mode: force pump increases pressure automatically
Reducing pressure mode: self-motion ladder reducing pressure mode
SpO
2
(optional)
Measure range: 35%~100%
Accuracy: ±2%, 70%~100%
SpO
2
Probe Pulse Rate:
Measuring range:30bpm~250bpm
Resolution:1bpm
Display: 2.8" High-definition color LCD
Power: Four "AA" Batteries/5V or 6V Power adapter
Safety character:ClassⅡ Type BF
Physics speciality
Dimension: 130mm*110mm*80 mm
Weight: 300g (No Batteries)
Accessories
Cuff for adult
Cuff for child
Cuff for infant
Cuff for Neonate(Ship from China)
Digital Integrative SpO2 Probe (optional)
User manual
Software CD
USB data line
Power adapter(optional)
Software function
PC software is connect to the device by USB interface
Can download NIBP measure result of the terminal device
Can display trend graph,histogram, pie chart,etc.
Can edit every piece of NIBP data, and add annotation to it.
Can edit basic information,doctor's advice information, NIBP status instruction, current medicine-taken information, etc.
Support print preview,print the report.
Buy safe Products
The following FDA Disclaimer is required for all eBay listing in Healthcare category and is included for
REFERENCE:
The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state
and local regulatory agencies.If the item is subject to FDA regulation, We will verify your status as an
authorized purchaser of this item before shipping of the item.
If you have questions about legal obligations regarding sales of medical devices, you should consult with
the FDA's Center for Devices and Radiological Health.
The Fingertip Pulse Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with
the code 197923,
and certified by FDA of United States and CE,TUV of Europe.The Fingertip Pulse
Oximeter that is FDA 510K Approved.
Only English user guide,if you need any other language,please contact us!
Track Page Views With
Auctiva's FREE Counter