-40%
24 Hour Ambulatory Blood Pressure Monitor NIBP Holter USB Software,US Seller,FDA
$ 62.83
- Description
- Size Guide
Description
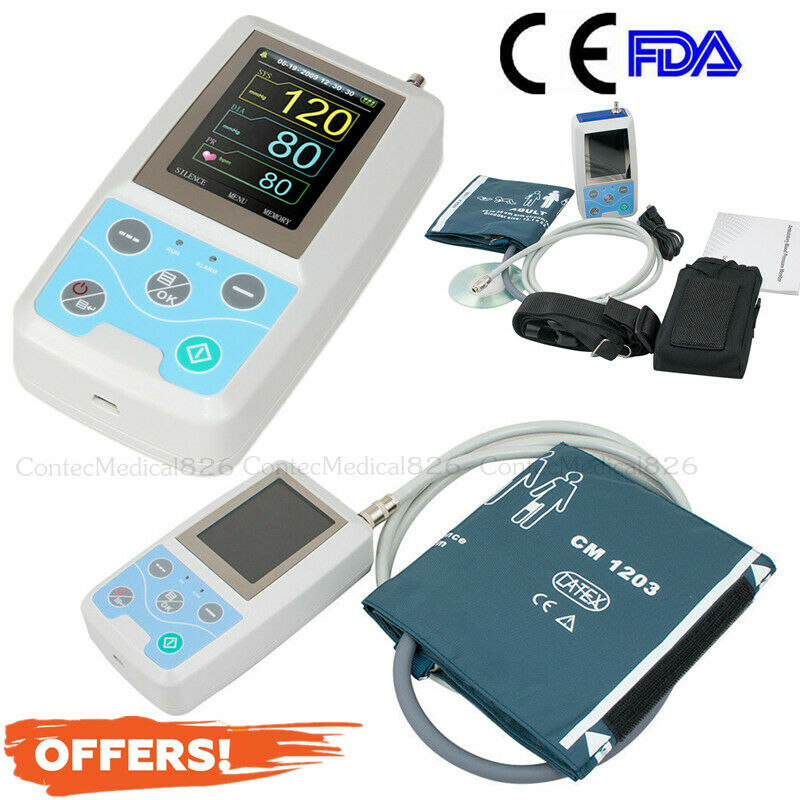
Test ItemABPM50 24 Hours Ambulatory Blood Pressure Monitor
NIBP Holter + Software+USB
Description
Introduction
ABPM50 is a handhold ambulatory blood pressure monitor which is designed according to oscillography principle and could 24 hours ambulatory monitor NIBP.It supplies the exact estimation warrant, and applies to clinique, the hospital wards or family daily health care.
Features
Compact and portable,friendly interface, easy to use
Patient range: adult, pediatric, neonatal
24 hours ambulatory NIBP measure function,at best 350 pieces of ambulatory NIBP measure data can be recorded once.
Automatic measure and manual measure of the perfect combination,at best 300 pieces of NIBP measure data can be recorded once by manual measure.
High-definition color TFT display, strong visibility
By data review interface such as "data list","trend graph","big font", NIBP data is clear at a glance
The device can display low power prompt information, alarm information, error information and time information richly
Supply two kinds of NIBP measure unit: mmHg / kPa
Display interface can be switched between Chinese and English
Parameter alarm dispose function is optional
Communicate with PC,PC software can achieve data review, analysis measure results,seeing trend graph, printing reports and other functions
Performance
NIBP
Measure Method: Oscillometry
Measure Mode: The upper arm measure
Automatic Measure Interval: 15、30、60、120、240 minutes
Measure range: 0kPa(0mmHg)~38.67kPa(290mmHg)
Resolution: 1mmHg
Accuracy: ±3mmHg
Pulse Rate
Measure range:40bpm~240bpm
Resolution:1bpm
Display: 2.4" TFT colour LCD
Alarm parameter: SYS / DIA
Increasing pressure mode: force pump increases pressure automatically
Reducing pressure mode: self-motion ladder reducing pressure mode
Power: DC 3V(Two "AA",1.5V Alkali Battery)
Product safety type:Type BF applied part (Internally powered,defibrillation-proof)
Physics speciality
Dimension: 128mm*69mm*36 mm (No including Packing)
Weight: <300g (Including Batteries)
Accessories
Cuff for adult
Disk (PC software)
User manual
USB data line
Pack
Software function
PC software is connect to the device by USB interface
Can download NIBP measure result of the terminal device
Can display scoop-shape trend graph,filling-type trend graph, histogram, pie chart, correlation line graph.
Can edit every piece of NIBP data, and add annotation to it.
Can edit basic information,doctor's advice information, NIBP status instruction, current medicine-taken information, etc.
Support print preview,print the report.
Payment
1.Paypal account
Shipping
Clearance: we will ship your item to your confirmed address.
Buyers' responsibility to pay duties,taxes and other extra charges by the government in your country.
Terms of Sale
Buy safe Products
The following FDA Disclaimer is required for all eBay listing in Healthcare category and is included for REFERENCE:
The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies.If the item is subject to FDA regulation, We will verify your status as an authorized purchaser of this item before shipping of the item.
If you have questions about legal obligations regarding sales of medical devices, you should consult with the FDA's Center for Devices and Radiological Health.
The Fingertip Pulse Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with the code 197923, and certified by FDA of United States and CE,TUV of Europe.The Fingertip Pulse Oximeter that is FDA 510K Approved
About Us
Contec Medical Systems Co.,Ltd; 20 Years manufacturer,we have stock in USA and China.
Only English user guide, if you need any other language, please contact us!
Only English user guide, if you need any other language, please contact us!